Shandong Xinbo was invited to attend the Chinese Society of Toxicology Conference on Drug Toxicology and Safety Evaluation (2023) and the Second Summit Forum of the Biomedical Industry in the Guangdon
2023/06/13

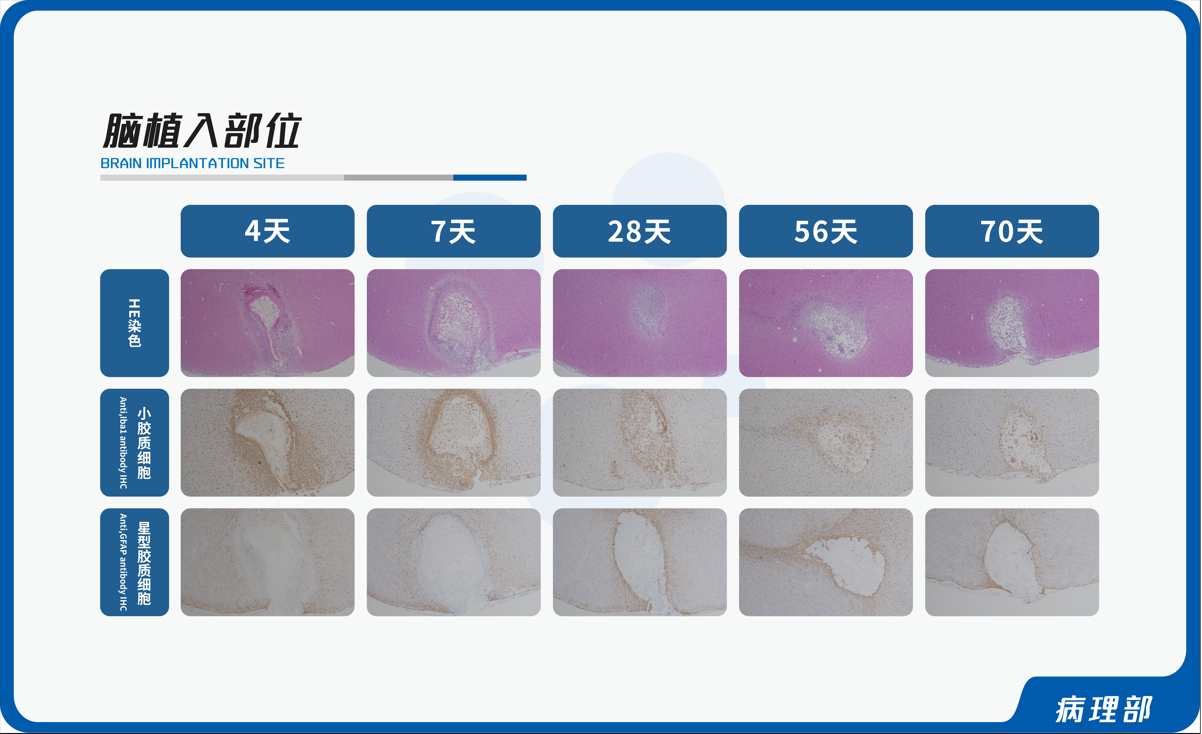
From June 15th to 18th, 2023, the "Chinese Society of Toxicology Drug Toxicology and Safety Evaluation Academic Conference (2023) and the Second Summit Forum of the Biomedical Industry in the Guangdong Hong Kong Macao Greater Bay Area" will be held at the Grand Yuntian International Hotel in Pingshan, Shenzhen. Shandong Xinbo Pharmaceutical R&D Co., Ltd. was invited to participate in this grand event. On the afternoon of June 17th, Li Yan from the Pathology Department gave a speech on the theme of "Organ Pathology Evaluation Methods for Animal Brain Implantation Experiments". We sincerely invite colleagues in the industry to attend this conference and communicate with the guests.

• Master of Veterinary Medicine
• Practicing veterinarian
• Advanced PI for pathological diagnosis
• Since 2011, I have been engaged in preclinical safety evaluation research of drugs, conducting reproductive toxicity research for 5 years, QA work for 1 year, and toxicity pathology research for 6 years
Shandong Xinbo Pharmaceutical Research Co., Ltd. was established in April 2010. It is a national high-tech enterprise, Shandong Enterprise Technology Center, and Shandong Gazelle Enterprise. It is the only scientific research service-oriented enterprise in the province that integrates pharmacological research, toxicology research, drug analysis and testing services, animal pathology testing, and medical device evaluation services.
Under the guidance of pathology expert Lang Shuhui, the Pathology Diagnosis Laboratory of Shandong Xinbo Pharmaceutical Research Co., Ltd. has obtained the "Toxicity Pathology Examination" capability verification certificate organized by the China National Accreditation Service for Conformity Assessment, marking the recognition of Shandong Xinbo Pharmaceutical Research Co., Ltd.'s professional ability in toxicity pathology diagnosis by the China National Accreditation Service for Conformity Assessment. The test report issued by the laboratory has authority and credibility.

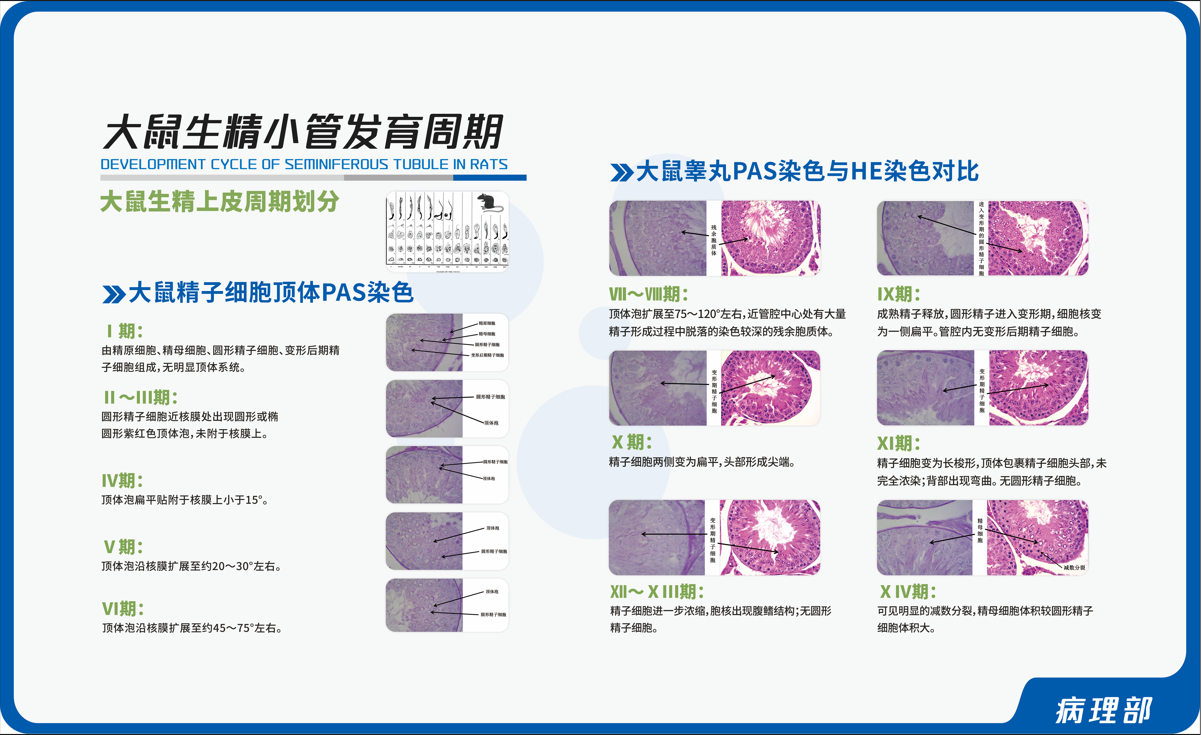
The company has established multiple sampling techniques and special staining techniques for preclinical evaluation of various drugs and medical devices.
Sampling techniques: Establish standardized sampling techniques for commonly used experimental animals, and develop special sampling techniques for small pig and monkey neurotoxicity tests, medical device related parts (skin, muscle, internal organs, bones, brain implants), special medication parts (nasal cavity, ears, etc.), animal tissue chips, etc. based on experimental characteristics.
Staining techniques: Conventional HE staining, as well as various immunohistochemical staining and special chemical staining techniques (such as periodic acid Schiff's staining, Masson trichrome staining, Mallory phosphotungstic acid hematoxylin staining, starch staining, toluidine blue staining, oil red O staining, etc.) can be performed.