Shandong Xinbo helps Jino's sanitary reorganization with shingles vaccine (CHO cells) obtained clinical clinical
2024/04/02
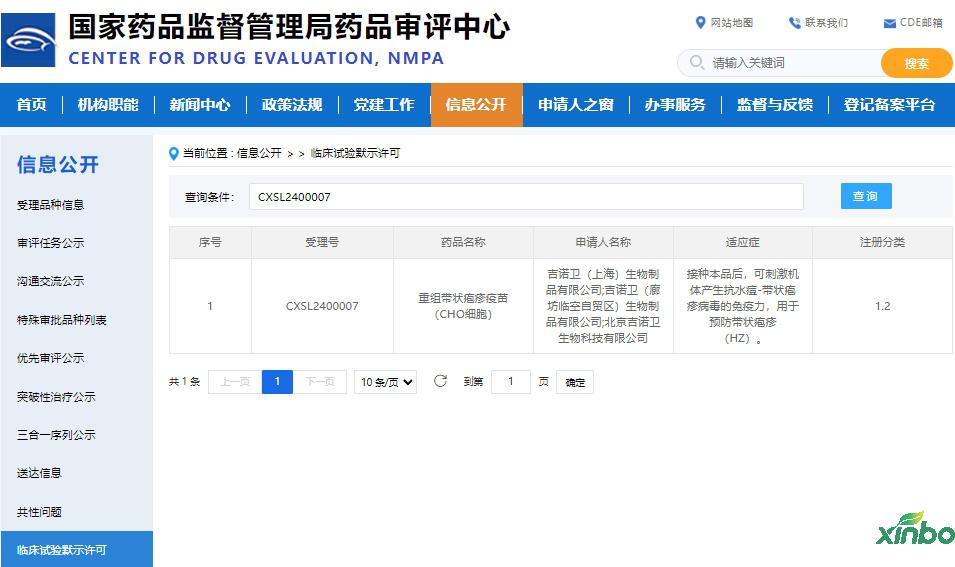
On March 29, 2024, the 1.2 type of biological products independently developed by Jino Synchry-Reorganizing Bandbite Vaccine (CHO cells) obtained the notice of approval of the drug clinical trial of the State Drug Administration (Acceptance Number: CXSL2400007), which is used clinically for it Prevent shingles (Hz). On January 27, 2024, this product has obtained permission to apply for clinical trial applications for new drugs of the US Food and Drug Administration (FDA).
"Consensus of Chinese Singer Vaccine Prevention Experts in 2022" recommends people who are 50 years old and over the age of 50 and have normal immune function. According to the statistics of Fhstana, the in vaccination rate of zostering in the age of 50 and above in China is 0.1%, 5.2%in the European Union and 26.8%in the United States. Growth space.
At present, the only shingles reorganized protein vaccine in the global market is the shingrix (domestic name "Xin An Li Shi"), a GSK GSK was approved by the FDA in 2017. This product has exceeded US $ 3.6 billion globally in 2022, and is the fifth largest best -selling vaccine product in the world. At present, China has not yet reorganized VZV (shingles) protein vaccines for listing.
The reorganized VZV protein vaccine independently developed by Jino Sanitation uses the double -sediment technology platform with independent intellectual property rights, as well as independent intelligent design and screening GE di -gymnarian fusion protein. The safety, cellular immune level, and body fluid immunity of this product are consistent with Shingrix in clinical animal experiments.
Part of the content is quoted from:
https://www.genevax.com.cn/newsinfo/6818959.html
Shandong Xinbo Pharmaceutical Research Co., Ltd. has rich experience in the pre -clinical safety evaluation of biological products. It can carry out pre -clinical research on preventive vaccines and treatment of vaccines, including overall method science establishment, specific indicators testing, pharmaceutical dynamic power power Learn test testing, full -set clinical safety evaluation.
At present, the varieties that have been carried out are inactivated seedlings, poisoning seedlings, reorganized seedlings, and nucleic acid seedlings, covering hand, foot, and mouth disease vaccine, wheel -like virus vaccine, hepatitis virus vaccine, papilloma virus vaccine, rabies vaccine, AIDS vaccine, Baibai broken break Vaccium, rubella vaccine, influenza vaccine, respiratory hypotomia vaccine, coronary virus vaccine, shingles vaccine, etc., and the evaluation of new agents have helped many biological products to obtain clinical trial applications.
Business Inquiry Tel: 0534-5056588
Website: www.xinbo.cn
Email: bd@xinbo.cn
Address: No. 1308, Fumin Road, Linyi Economic Development Zone, Dezhou City, Shandong Province
Floor, Building A, Building A, Lishan International Cell Medicine Industrial Park, Jinan City, Shandong Province
The Drug Safety Evaluation Research Center of Shandong Xinbo Drug Research Co., Ltd. undertakes the comprehensive safety evaluation research pre -clinical safety evaluation of shingles vaccine (CHO cells), showing that this product has good safety and immunogenicity, helping them to obtain clinical trials license.
About shingles
Herpes Zoster is the infectious skin disease caused by the adequate skin disease caused by a long-term lurking in the back root ganglia or the cranial nerve festival for a long time. It is estimated that about 1/3 people will suffer from shingles in their lives, but about 1/2 of the people will suffer from shingles among people ≥85 years old."Consensus of Chinese Singer Vaccine Prevention Experts in 2022" recommends people who are 50 years old and over the age of 50 and have normal immune function. According to the statistics of Fhstana, the in vaccination rate of zostering in the age of 50 and above in China is 0.1%, 5.2%in the European Union and 26.8%in the United States. Growth space.
At present, the only shingles reorganized protein vaccine in the global market is the shingrix (domestic name "Xin An Li Shi"), a GSK GSK was approved by the FDA in 2017. This product has exceeded US $ 3.6 billion globally in 2022, and is the fifth largest best -selling vaccine product in the world. At present, China has not yet reorganized VZV (shingles) protein vaccines for listing.
The reorganized VZV protein vaccine independently developed by Jino Sanitation uses the double -sediment technology platform with independent intellectual property rights, as well as independent intelligent design and screening GE di -gymnarian fusion protein. The safety, cellular immune level, and body fluid immunity of this product are consistent with Shingrix in clinical animal experiments.
Part of the content is quoted from:
https://www.genevax.com.cn/newsinfo/6818959.html
About Shandong Xinbo
Shandong Xinbo Pharmaceutical Research Co., Ltd. has rich experience in the pre -clinical safety evaluation of biological products. It can carry out pre -clinical research on preventive vaccines and treatment of vaccines, including overall method science establishment, specific indicators testing, pharmaceutical dynamic power power Learn test testing, full -set clinical safety evaluation.
At present, the varieties that have been carried out are inactivated seedlings, poisoning seedlings, reorganized seedlings, and nucleic acid seedlings, covering hand, foot, and mouth disease vaccine, wheel -like virus vaccine, hepatitis virus vaccine, papilloma virus vaccine, rabies vaccine, AIDS vaccine, Baibai broken break Vaccium, rubella vaccine, influenza vaccine, respiratory hypotomia vaccine, coronary virus vaccine, shingles vaccine, etc., and the evaluation of new agents have helped many biological products to obtain clinical trial applications.
Business Inquiry Tel: 0534-5056588
Website: www.xinbo.cn
Email: bd@xinbo.cn
Address: No. 1308, Fumin Road, Linyi Economic Development Zone, Dezhou City, Shandong Province
Floor, Building A, Building A, Lishan International Cell Medicine Industrial Park, Jinan City, Shandong Province
