Video playback │ The drug review center held the seventh "drug review cloud class" in 2024
2024/08/13
Original link:
https://www.cde.org.cn/main/newinfocommon/8ADE9E83F7D419530AFD34B5D644A
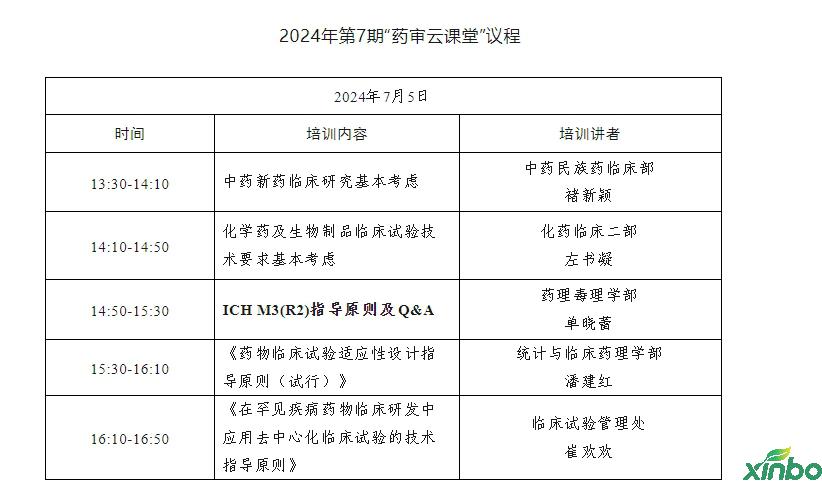
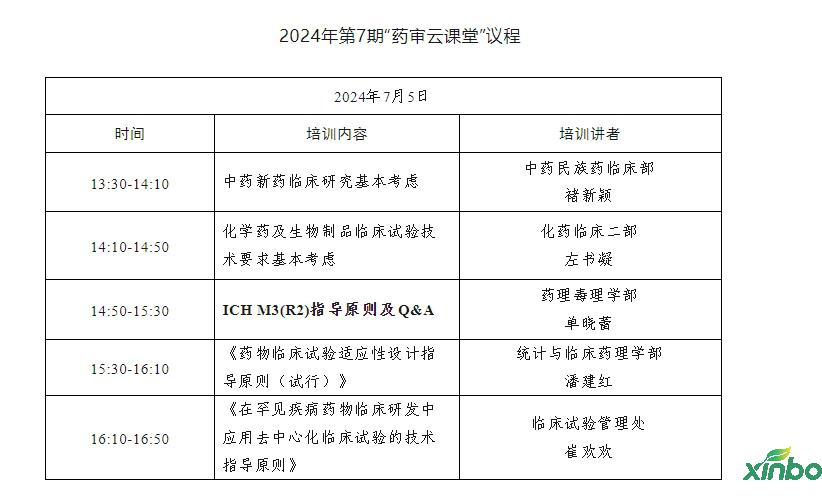
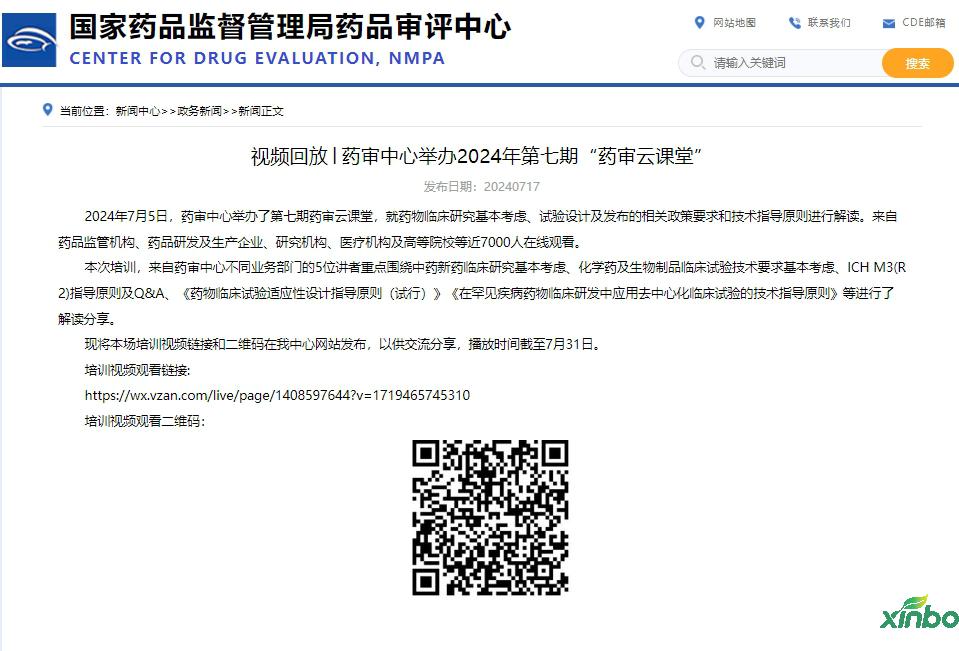
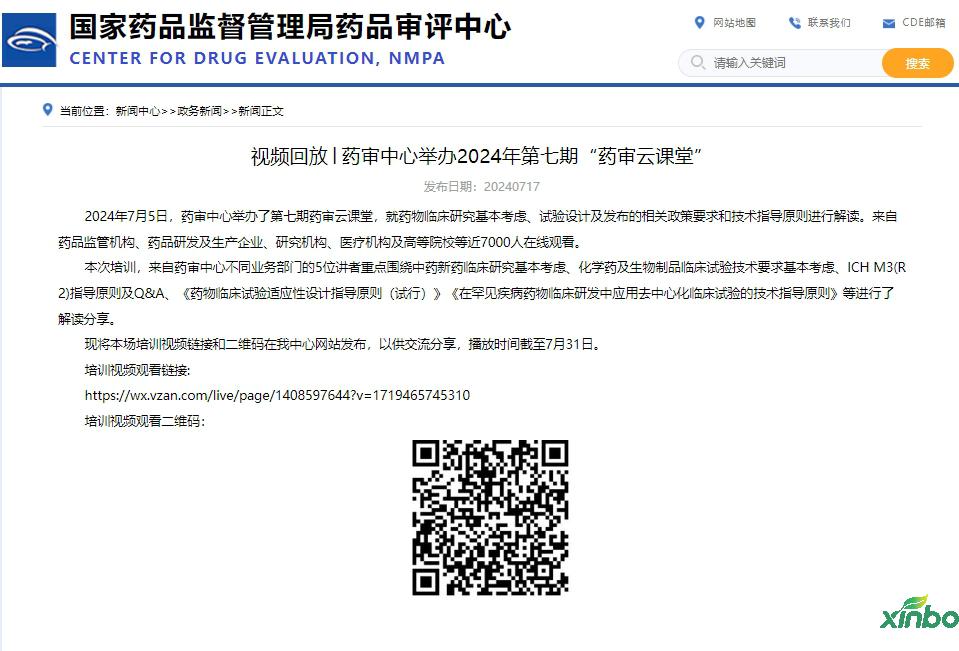
On July 5, 2024, the Pharmaceutical Audit Center held the seventh pharmaceutical review classroom to interpret the basic policy requirements and technical guidance principles of the basic consideration, test design and release of drug clinical research. Nearly 7,000 people from drug regulatory agencies, pharmaceutical research and development and production enterprises, research institutions, medical institutions, and colleges and universities are watching online.
In this training, the five lectures from different business departments from the Medical Examination Center are: Chu Xinxin, Chu Xinxin, the Ministry of Hydinology of the Chinese Medicine National Pharmaceuticals, the two left books of the clinical department of chemical drugs, Shan Xiaolei, the Department of Pharmacology, Pharmacology, Pan Jianhong, the Department of Statistics and Clinical Pharmacology, and clinical clinical clinical. Cui Huanhuan, the Test Management Office, focuses on the basic considerations of clinical research on new medicines of traditional Chinese medicine, basic considerations of chemical drugs and clinical trial technology requirements for biological products, the principles of ICH M3 (R2) guidance, and Q & A, "Principles of Drug Clinical Test Design Guidance (Trial)", "Trial)" "" The technical guidance principles of decentralized clinical trials in the clinical research and development of rare diseases of the disease "and other solutions and sharing were solved.
This training video link and QR code are released on our center website for communication and sharing. The playback time is as of July 31.
Training video viewing link:
https://wx.vzan.com/live/page/1408597644?v=1719465745310
Training video Watch QR code: