Academic Report | Shandong Xinbo Special Session - Experimental Pathology Public Welfare Online Learning Conference
2024/09/23
1、 Meeting content (sorted by report order)

2、 Registration method
1. Scan the code to register

2.Registration link:
https://p.baominggongju.com/share?eid=66eaf2593148d8a16308000d&referer=odVL41H21KLfydbPZuzp4Kv_8OS8
三、About Xinbo

Shandong Xinbo Pharmaceutical Research Co., Ltd. is a scientific research service-oriented enterprise that integrates pharmacological research, toxicology research, drug analysis and testing services, animal pathology testing, and medical device evaluation services. It is a high-tech enterprise, Shandong Province Enterprise Technology Center, and Shandong Province Gazelle Enterprise.
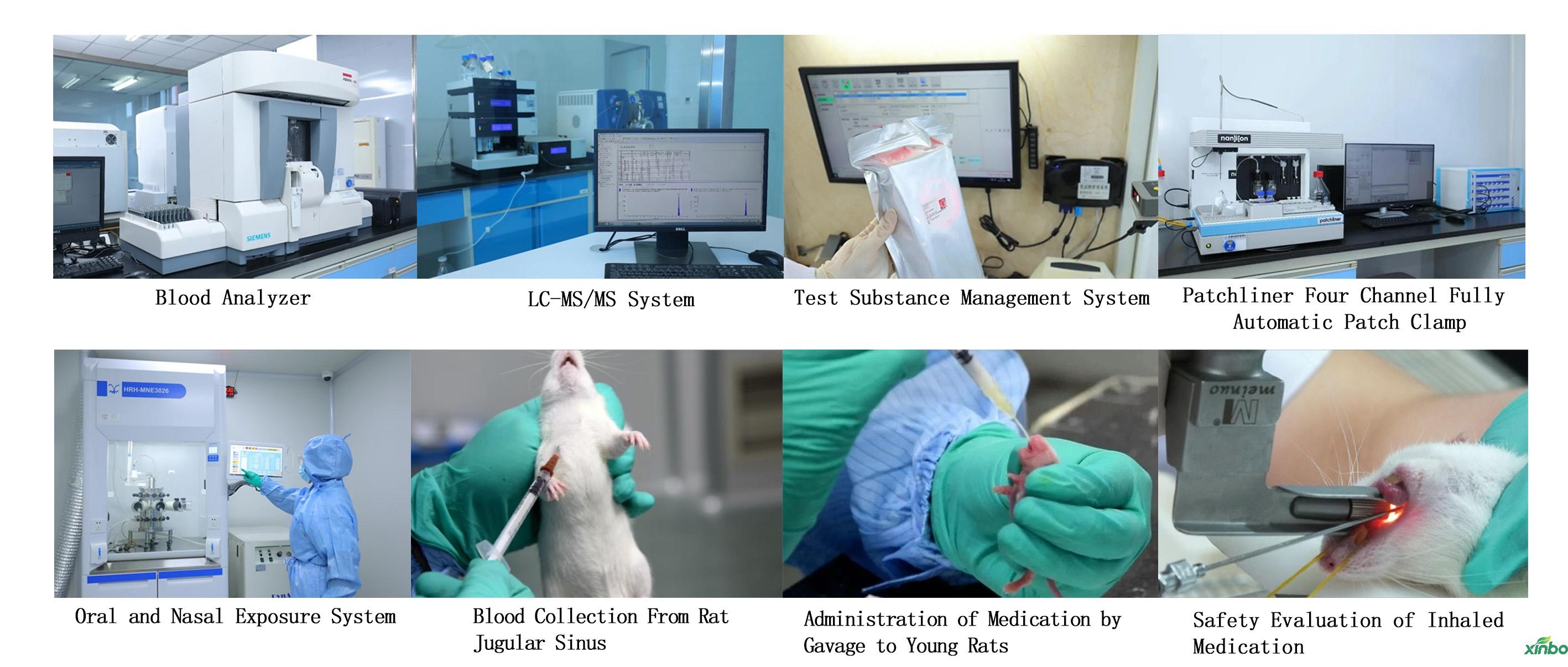
The company is located in the Beijing Tianjin Hebei Shandong economic zone, and has established a pilot base in the Linyi Economic Development Zone of Dezhou City, known as the "Nine Dash Tianqu" and "Gateway to Shenjing". At present, more than 16000 square meters of pharmacology and toxicology research laboratories that meet international standards have been built, equipped with more than 800 advanced instruments and equipment at home and abroad. Among them, the toxicology research laboratory can conduct 9 studies including single dose toxicity tests, repeated dose toxicity tests, safety pharmacology tests, reproductive toxicity tests, genetic toxicity tests, local toxicity tests, immunogenicity tests, toxicokinetics tests, and carcinogenicity tests in accordance with the GLP standards of NMPA and FDA.
The company is committed to providing high-quality commissioned research services for new drug and medical research and development institutions. Since its establishment in 2010, it has provided over 10000 services to more than 540 pharmaceutical companies, new drug research institutions, and research institutes both domestically and internationally. We have completed preclinical evaluation services for nearly 400 innovative drugs in accordance with domestic and foreign regulatory requirements, including small molecule drugs, biomacromolecule drugs, new preventive and therapeutic vaccines, and traditional Chinese medicine/natural medicine evaluation work. We have helped research and development units obtain more than 50 clinical approvals for new drugs, especially in the evaluation of rapidly developing therapeutic vaccines, cell and gene therapy drugs in recent years, and have accumulated rich experience. Completed the evaluation of nearly 4000 varieties of generic drugs.
In addition, the company continuously innovates its practices and breaks through barriers, improving and exploring difficult areas such as administration technology for young animals, continuous intravenous administration technology, and tracheal administration technology, and maturely applying them to safety evaluation. The company also pays attention to the demand for innovative development in the global pharmaceutical industry, focusing on the key link of innovative drug research and development. With the accumulated experience in serving the domestic and international pharmaceutical industry, it provides comprehensive preclinical research and development services for new drugs in the global pharmaceutical industry.