According to the official website of the Center for Drug Evaluation (CDE) of the China National Medical Products Administration and public information, in 2024, Shandong Xinbo Pharmaceutical Research Co., Ltd. (hereinafter referred to as "Shandong Xinbo") assisted Beijing Yingkerui Innovative Medicine Co., Ltd. in obtaining implied clinical trial permits (IND) for two Class 1.1 innovative Chinese medicine drugs, namely "Shendai Rectal Suppository" and "Shengge Granules".
As an important partner in the research and development process, the Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. strictly follows the guidelines of NMPA and ICH. With its rich project experience and mature technical platform, it has undertaken preclinical safety evaluation research for the two drugs mentioned above, helping partners to obtain clinical trial permits smoothly.
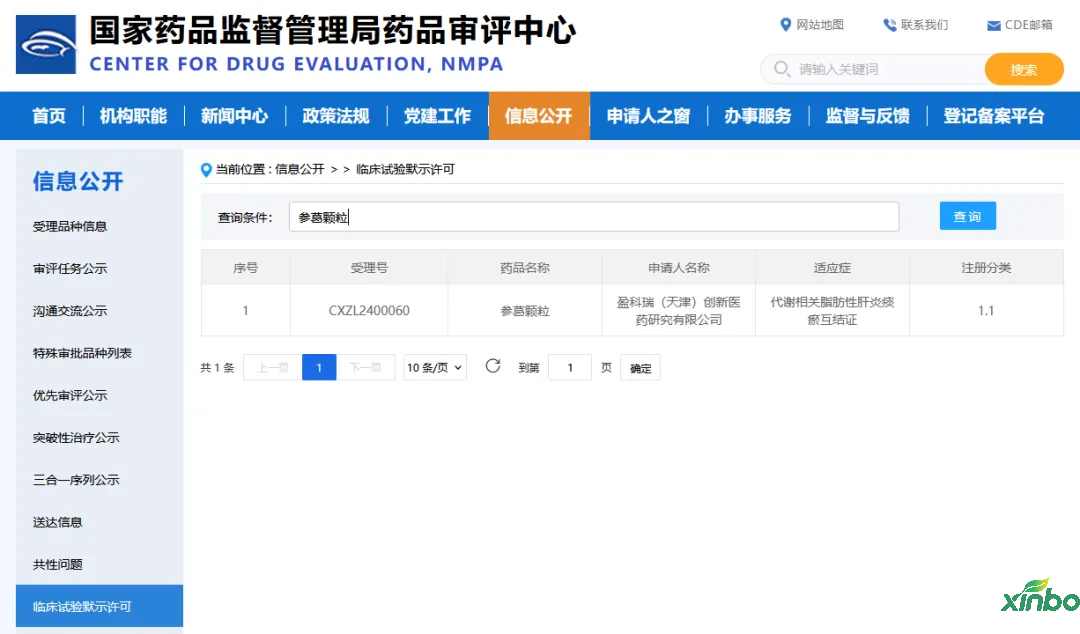
Ginseng granules
Shen Ge Fang is an empirical formula formed on the basis of tonifying the kidney and strengthening the spleen. Through undertaking a clinical research project of the Shanghai Science and Technology Commission, it has been further confirmed that Shen Ge Fang has significant clinical efficacy, safety, and no toxic side effects.
Shen Ge granules have the effects of invigorating the spleen, promoting diuresis, and removing phlegm and blood stasis. Used for patients with metabolic related fatty hepatitis and phlegm stasis syndrome, the symptoms include pain in the lower right rib area or rib, aversion to oil, tightness in the chest and epigastric area, dull complexion, pale and dull tongue, bruises on the edges, greasy coating, and smooth or astringent pulse. The combination of all ingredients has the effects of strengthening the spleen, eliminating dampness, and removing phlegm and blood stasis.
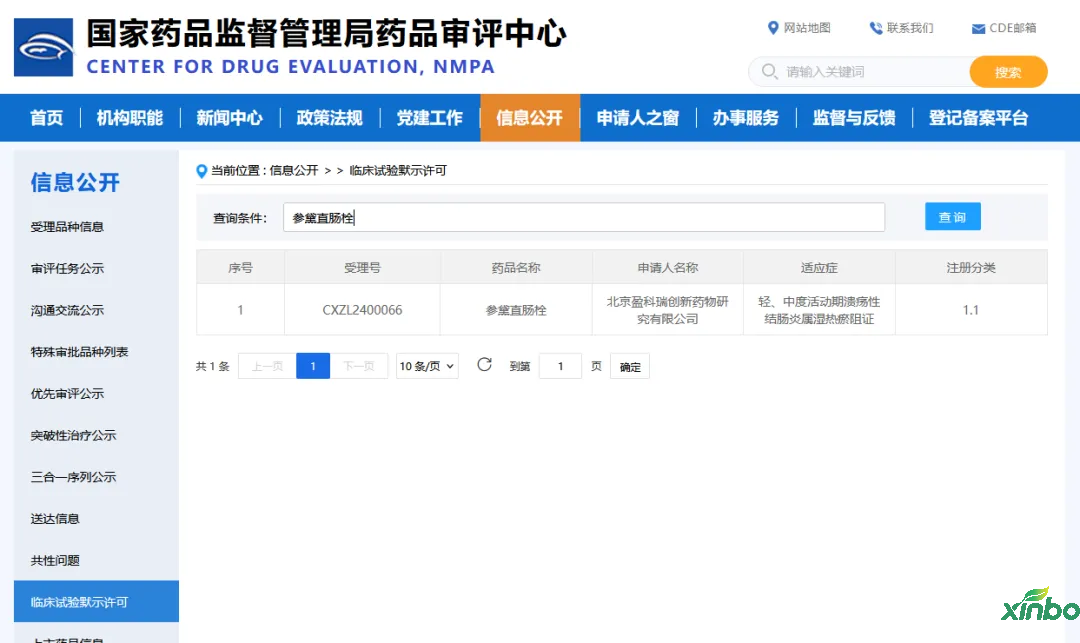
Shen Dai rectal suppository
Ulcerative colitis (UC) is a chronic, non-specific inflammation affecting the rectum and colon. Based on its main clinical manifestations such as abdominal pain, diarrhea, mucous purulent stools, and severe diarrhea, it can be classified as a traditional Chinese medicine syndrome such as "chronic dysentery", "resting dysentery", and "intestinal depression". During the active phase of UC, the disease is located in the intestine and is characterized by a combination of deficiency and excess. The weakness of the spleen and stomach is the main cause, with dampness (heat), stasis heat, heat toxicity, phlegm turbidity, qi stagnation, and blood stasis as the indicators. The onset of UC is caused by deficiency leading to excess, ultimately resulting in a process of mixed deficiency and excess.
The Shendai rectal suppository declared by Beijing Yingkerui is intended for patients with mild to moderate active ulcerative colitis who belong to the damp heat and blood stasis syndrome. It has the functions of clearing heat and dampness, cooling blood to stop bleeding, astringing ulcers and generating muscle. It is used to treat mild to moderate active ulcerative colitis who belong to the damp heat and blood stasis syndrome. The approval of this clinical trial brings new drugs for the treatment of ulcerative colitis.


