Shandong Xinbo assisted in the clinical approval of BPL BioPharma's Twinrix (two-component) hepatitis B and tetanus conjugate vaccine for adults and adolescents
2025/11/18
As a key partner in the R&D process, Shandong Xinbo strictly adheres to the guidance principles of NMPA and ICH. Leveraging its profound expertise in preclinical safety evaluation, the company provides a comprehensive suite of preclinical safety assessment technologies for the development of this innovative drug, facilitating partners in successfully obtaining clinical trial approvals.

One vaccine prevents three diseases and fills the gap in immunity
For a long time, the protection against pertussis, diphtheria, and tetanus in adults and adolescents has been neglected, and the supply of related vaccines is insufficient. The vaccine approved by Baike Biotechnology this time targets the population aged 10 years and above with precision. Through the efficient design of "one vaccine to prevent three diseases", vaccination can activate the body's immune response and directly resist invasive infections caused by three infectious diseases - whether it is pertussis, which may cause severe coughing, diphtheria, which can damage the respiratory tract, or tetanus, which may cause muscle rigidity, it can form effective protection.
Xinbo makes efforts: Pre clinical research tailored to ensure safety
As an important partner of Baike Biotechnology, Shandong Xinbo has conducted refined preclinical research on the "suitability of the drug population" of the vaccine: relying on the national GLP certification platform, a comprehensive toxicological evaluation of the vaccine components has been conducted to ensure safety for adolescents and adults; Based on the physiological characteristics of adults and adolescents, design targeted experimental plans to provide scientific basis for subsequent clinical trial design.
Collaborative win-win: Continuously tackling challenges to safeguard public health
The cooperation between Shandong Xinbo and Baike Biotechnology has formed a "1+1>2" effect. The clinical approval of the Bai Bai Tuo vaccine this time is another achievement of deep collaboration between the two sides in the field of vaccine research and development. In the future, Shandong Xinbo will continue to leverage its preclinical research advantages and work together with industry partners such as Baike Biotechnology to focus on more unmet medical needs, allowing more safe and effective vaccines to enter public life and build a solid defense line for public health.
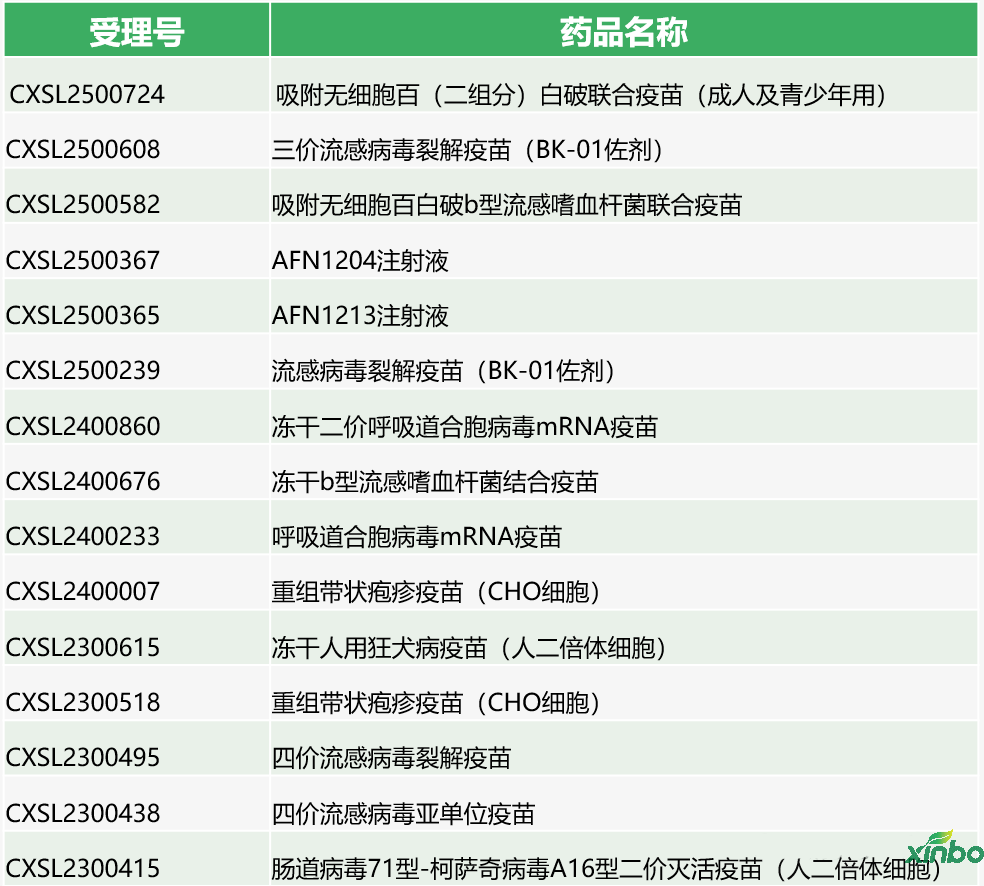
Only show part
- Pre:Shandong Xinbo assists Qingdao Weineng Biotech in obtaining clinical approval for its innovative stem cell drug, tackling two major challenges: AIDS and diabetic kidney disease!
- Next:Shandong Xinbo helped Ruiji Biology to obtain the approval of the first freeze-dried tuberculosis mRNA vaccine in the world
